 Work, work, work. You might head off to your job one day, sit at a computer, and type away at the keys. That's all we do here. Is that work? To a physicist, only parts of it are. Work is done when aforce that is applied to an object movesthat object. The work is calculated by multiplying the force by the amount of movement of an object (W = F * d). A force of 10 newtons, that moves an object 3 meters, does 30 n-m of work. A newton-meter is the same thing as a joule, so the units for work are the same as those for energy – joules.
Work, work, work. You might head off to your job one day, sit at a computer, and type away at the keys. That's all we do here. Is that work? To a physicist, only parts of it are. Work is done when aforce that is applied to an object movesthat object. The work is calculated by multiplying the force by the amount of movement of an object (W = F * d). A force of 10 newtons, that moves an object 3 meters, does 30 n-m of work. A newton-meter is the same thing as a joule, so the units for work are the same as those for energy – joules. Sitting and looking at a computer screen is not work. Tapping on the keyboard and making the keys move is work. Your fingers are applying a force and moving the keys. Driving to your job is not work because you just sit, but the energy your car engine uses to move the car does work. You have to exert a force AND move something to qualify as doing work.
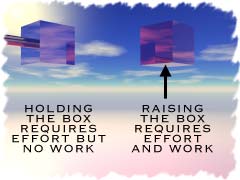 Imagine that you are holding a brick above the ground. Your arm is straight out in front of you and it's pretty tough to hold. Slowly, your arm gets tired, the brick feels heavier and heavier, and you finally have to stop to let your arm rest. Even though you put forth a lot of effort to hold the brick up, did you do any work on the brick? Nope. The brick didn't move. No work was done if no movement happened. If you lifted the brick again after your arm had rested, that would be work.
Imagine that you are holding a brick above the ground. Your arm is straight out in front of you and it's pretty tough to hold. Slowly, your arm gets tired, the brick feels heavier and heavier, and you finally have to stop to let your arm rest. Even though you put forth a lot of effort to hold the brick up, did you do any work on the brick? Nope. The brick didn't move. No work was done if no movement happened. If you lifted the brick again after your arm had rested, that would be work. Transfer of Energy
Work transfers energy from one object to another. We've already talked about moving objects. What else? Work is also linked to the expansion andcompression of gases. When a gas tries to expand, it exerts an increasing force on the surfaces of a container and may make those surfaces move. The gas would then be doing work and transferring energy to the container. If you heat a balloon (carefully), the molecules of air in the balloon gain energy and strike the inner walls of the balloon with greater force. Because the inner surface of the balloon is flexible, that surface moves outward. The air does work, and transfers energy to the balloon. If you compress a balloon, you do work, and transfer energy to the air inside the balloon.Measuring Work for Gases
When scientists measure the work done on, or by, gases, they look at the system at the beginning and the end of the project. They look at the initial and final states. To figure out the total work done on, or by, a gas system, they use the formula W = P (delta)V. W stands for work, P is the pressure of the system (for gases), and delta V is the change in volume for the system. A variation would be W = V(delta P), where V is volume, and delta P is the change in pressure. The delta values are taken at the beginning and end.Sometimes they might take measurements while things are happening. Those are measurements of intermediate states. They could then use the intermediate measurements to calculate work, and then total those work values up to figure the total work done.








0 comments:
Post a Comment