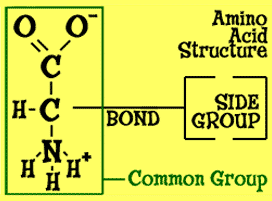
 Amino acids have a two-carbon bond. One of the carbons is part of a group called the carboxyl group (COO-). A carboxyl group is made up of one carbon (C) and two oxygen (O) atoms. That carboxyl group has a negative charge, since it is a carboxylic acid (-COOH) that has lost itshydrogen (H) atom. What is left — the carboxyl group — is called a conjugate base. The second carbon is connected to the amino group. Amino means there is an NH2 group bonded to the carbonatom. In the image, you see a "+" and a "-". Those positive and negative signs are there because, in amino acids, one hydrogen atom moves to the other end of the molecule. An extra "H" gives you a positive charge.
Amino acids have a two-carbon bond. One of the carbons is part of a group called the carboxyl group (COO-). A carboxyl group is made up of one carbon (C) and two oxygen (O) atoms. That carboxyl group has a negative charge, since it is a carboxylic acid (-COOH) that has lost itshydrogen (H) atom. What is left — the carboxyl group — is called a conjugate base. The second carbon is connected to the amino group. Amino means there is an NH2 group bonded to the carbonatom. In the image, you see a "+" and a "-". Those positive and negative signs are there because, in amino acids, one hydrogen atom moves to the other end of the molecule. An extra "H" gives you a positive charge. Making Chains
Even though scientists have discovered over 50 amino acids, only 20 are used to make something called proteins in your body. Of those twenty, nine are defined as essential. The other eleven can be synthesized by an adult body. Thousands of combinations of those twenty are used to make all of the proteins in your body. Amino acids bond together to make long chains. Those long chains of amino acids are also called proteins.Essential Amino Acids: Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, and Valine.
Nonessential Amino Acids: Alanine, Asparagine, Aspartic Acid, Glutamic Acid.
Conditional Amino Acids: Arginine (essential in children, not in adults), Cysteine, Glutamine, Glycine, Proline, Serine, and Tyrosine.
Something Called Side Groups
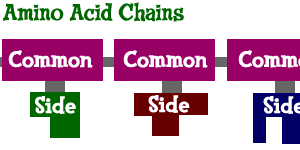 The side groups are what make each amino acid different from the others. Of the 20 side groups used to make proteins, there are two main groups: polar and non-polar. These names refer to the way the side groups, sometimes called "R" groups, interact with the environment. Polar amino acids like to adjust themselves in a certain direction. Non-polar amino acids don't really care what's going on around them. The polar and nonpolar chemical traits allow amino acids to point towards water (hydrophilic) or away from water (hydrophobic). The growing chains can then begin to twist and turn when they are being synthesized.
The side groups are what make each amino acid different from the others. Of the 20 side groups used to make proteins, there are two main groups: polar and non-polar. These names refer to the way the side groups, sometimes called "R" groups, interact with the environment. Polar amino acids like to adjust themselves in a certain direction. Non-polar amino acids don't really care what's going on around them. The polar and nonpolar chemical traits allow amino acids to point towards water (hydrophilic) or away from water (hydrophobic). The growing chains can then begin to twist and turn when they are being synthesized. 







0 comments:
Post a Comment