There are two main types of chemical bonds that hold atoms together: covalent and ionic/electrovalent bonds. Atoms that share electrons in a chemical bond have covalent bonds. An oxygen molecule (O2) is a good example of a molecule with a covalent bond. Ionic bonds occur when electrons are donated from one atom to another. Table salt (NaCl) is a common example of a compound with an ionic bond.
You may also learn about a third type of bond. Metallic bonds occur between metal atoms. We’re going to focus on ionic and covalent bonds.
Physical and Chemical Traits of Compounds
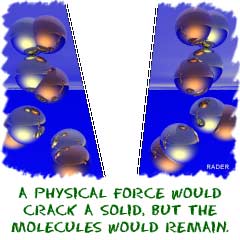 When we discuss phase changes inmatter, we are looking at physicalchanges. Physical forces alone (unless you're inside of the Sun or something extreme) rarely break down compounds completely. You can apply heat to melt an ice cube, but there will be no change in the water molecules. You can also place a cup of water in a container and decrease the pressure. The water will eventually boil, but the molecules will not change.
When we discuss phase changes inmatter, we are looking at physicalchanges. Physical forces alone (unless you're inside of the Sun or something extreme) rarely break down compounds completely. You can apply heat to melt an ice cube, but there will be no change in the water molecules. You can also place a cup of water in a container and decrease the pressure. The water will eventually boil, but the molecules will not change. Chemical changes in compounds happen when chemical bonds are created or destroyed. Forces act on the bonds between atoms, changing the molecular structure of a substance. You can pour liquid acid on a solid and watch the solid dissolve. That process is a chemical change because molecular bonds are being created and destroyed. Geologists pour acids on rocks to test for certain compounds.
There are millions of different compounds around you. Probably everything you can see is one type of compound or another. When elements join and become compounds, they lose many of their individual traits. Sodium (Na) alone is very reactive. But when sodium and chlorine (Cl) combine, they form a non-reactive substance called sodium chloride (table salt, NaCl). New compounds have few or none of the physical or chemical traits of the original elements. They have a new life of their own.
Different Bonds Abound
If you look at sodium chloride, it is held together by one ionic/electrovalent bond. What about magnesium chloride (MgCl2)? It contains onemagnesium (Mg) and two chlorine (Cl) atoms. There are two ionic bonds. Methane (CH4) is made up of one carbon (C) and four hydrogen (H) atoms. There are four bonds and they are all covalent.Those examples have very simple chemical bonds. However, most compounds have combinations of ionic and covalent bonds. Let's look at sodium hydroxide (Na-OH)...
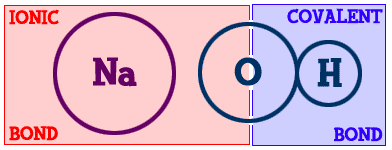
You can see the sodium (Na) part on the left and the hydroxide (-OH) part on the right. The bond that binds the hydrogen (H) to the oxygen (O) is covalent. The sodium is bonded to the hydroxide part of the compound with an ionic bond. This is a good example of how there can be different types of bonds within one compound.








0 comments:
Post a Comment