
The rate of a reaction is the speed at which a chemical reaction happens. If a reaction has a low rate, that means the molecules combine at a slower speed than a reaction with a high rate. Some reactions take hundreds, maybe even thousands, of years while others can happen in less than one second. If you want to think of a very slow reaction, think about how long it takes plants and ancient fish to become fossils (carbonization). The rate of reaction also depends on the type of molecules that are combining. If there are low concentrations of an essential element or compound, the reaction will be slower.
There is another big idea for rates of reaction called collision theory. The collision theory says that as more collisions in a system occur, there will bemore combinations of molecules bouncing into each other. If you have more possible combinations there is a higher chance that the molecules will complete the reaction. The reaction will happen faster which means the rate of that reaction will increase.
Think about how slowly molecules move in honey when compared to your soda even though they are both liquids. There are a lower number of collisions in the honey because of stronger intermolecular forces (forces between molecules). The greater forces mean that honey has a higher viscosity than the soda water.
Factors That Affect Rate
 Reactions happen - no matter what. Chemicals are always combining or breaking down. The reactions happen over and over, but not always at the same speed. A few things affect the overall speed of the reaction and the number of collisions that can occur.
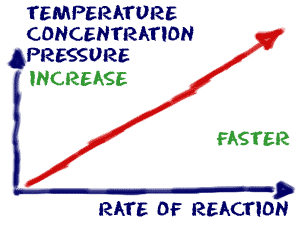
Reactions happen - no matter what. Chemicals are always combining or breaking down. The reactions happen over and over, but not always at the same speed. A few things affect the overall speed of the reaction and the number of collisions that can occur. Temperature: When you raise thetemperature of a system, the molecules bounce around a lot more. They have more energy. When they bounce around more, they are more likely to collide. That fact means they are also more likely to combine. When you lower the temperature, the molecules are slower and collide less. That temperature drop lowers the rate of the reaction. To the chemistry lab! Sometimes you will mix solutions in ice so that the temperature of the system stays cold and the rate of reaction is slower.
 Concentration: If there is more of a substance in a system, there is a greater chance that molecules will collide and speed up the rate of the reaction. If there is less of something, there will be fewer collisions and the reaction will probably happen at a slower speed. Sometimes, when you are in a chemistry lab, you will add one solution to another. When you want the rate of reaction to be slower, you will add only a few drops at a time instead of the entire beaker.
Concentration: If there is more of a substance in a system, there is a greater chance that molecules will collide and speed up the rate of the reaction. If there is less of something, there will be fewer collisions and the reaction will probably happen at a slower speed. Sometimes, when you are in a chemistry lab, you will add one solution to another. When you want the rate of reaction to be slower, you will add only a few drops at a time instead of the entire beaker. Pressure: Pressure: Pressure affects the rate of reaction, especially when you look at gases. When you increase the pressure, the molecules have less space in which they can move. That greater density of molecules increases the number of collisions. When you decrease the pressure, molecules don't hit each other as often and the rate of reaction decreases.
Pressure is also related to concentration and volume. By decreasing the volume available to the molecules of gas, you are increasing the concentration of molecules in a specific space. You should also remember that changing the pressure of a system only works well for gases. Generally, reaction rates for solids and liquids remain unaffected by increases in pressure.








0 comments:
Post a Comment