Elements as Building Blocks
The periodic table is organized like a big grid. Each element is placed in a specific location because of its atomic structure. As with any grid, the periodic table has rows (left to right) and columns (up and down). Each row and column has specific characteristics. For example, beryllium (Be) andmagnesium (Mg) are found in column two and share certain similarities while potassium (K) and calcium (Ca) from row four share different characteristics.You've got Your Periods...
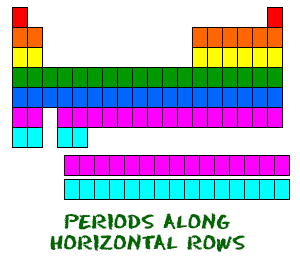 Even though they skip some squares in between, all of the rows read left to right. When you look at the periodic table, each row is called a period (Get it? Like PERIODic table.). All of the elements in a period have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. As you move down the table, every row adds an orbital. At this time, there is a maximum of seven electron orbitals.
Even though they skip some squares in between, all of the rows read left to right. When you look at the periodic table, each row is called a period (Get it? Like PERIODic table.). All of the elements in a period have the same number of atomic orbitals. For example, every element in the top row (the first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. As you move down the table, every row adds an orbital. At this time, there is a maximum of seven electron orbitals. ...and Your Groups
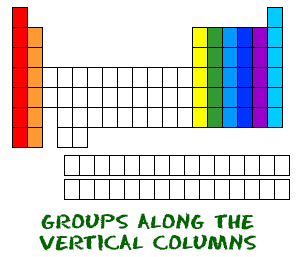 Now you know about periods going left to right. The periodic table also has a special name for its vertical columns. Each column is called a group. The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements.
Now you know about periods going left to right. The periodic table also has a special name for its vertical columns. Each column is called a group. The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements. Every element in the first column (group one) has one electron in its outer shell. Every element in the second column (group two) has two electrons in the outer shell. As you keep counting the columns, you'll know how many electrons are in the outer shell. There are exceptions to the order when you look at the transition elements, but you get the general idea. Transition elements add electrons to the second-to-last orbital.
For example, nitrogen (N) has the atomic number seven. The atomic number tells you there are seven electrons in a neutral atom of nitrogen. How many electrons are in its outer orbital? Nitrogen is in the fifteenth column, labelled 'Group VA'. The 'V' is the Roman numeral for five and represents the number of electrons in the outer orbital. All of that information tells you there are two electrons in the first orbital and five in the second (2-5).
Phosphorus (P) is also in Group VA which means it also has five electrons in its outer orbital. However, because the atomic number for phosphorus is fifteen, the electron configuration is 2-8-5.
Two at the Top
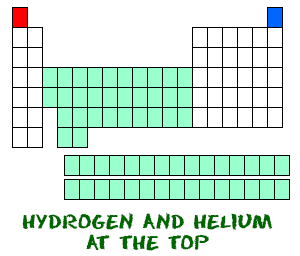 Hydrogen (H) and helium (He) are special elements. Hydrogen can have the electron traits of two groups: one and seven. For chemists, hydrogen is sometimes missing an electron like the members of group IA, and sometimes has an extra one as in group VIIA. When you study acids and bases you will regularly work with hydrogen cations (H+). A hydride is a hydrogen anion and has an extra electron (H-).
Hydrogen (H) and helium (He) are special elements. Hydrogen can have the electron traits of two groups: one and seven. For chemists, hydrogen is sometimes missing an electron like the members of group IA, and sometimes has an extra one as in group VIIA. When you study acids and bases you will regularly work with hydrogen cations (H+). A hydride is a hydrogen anion and has an extra electron (H-). Helium (He) is different from all of the other elements. It is very stable with only two electrons in its outer orbital (valence shell). Even though it only has two, it is still grouped with the noble gases that have eight electrons in their outermost orbitals. The noble gases and helium are all "happy," because their valence shell is full.








0 comments:
Post a Comment