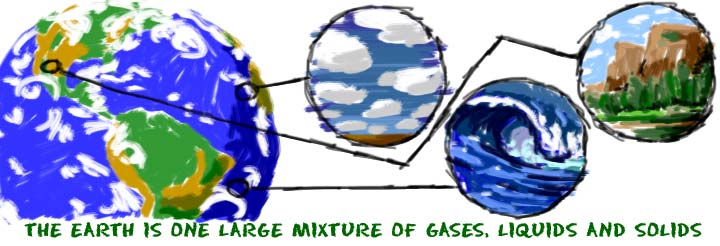
Even though matter can be found all over the Universe, you will only find it in a few forms on Earth. We cover five states of matter on the site. Each of those states is sometimes called a phase. There are many other states of matter that exist in extreme environments. Scientists will probably discover more states as we continue to explore the Universe.
Five States of Matter
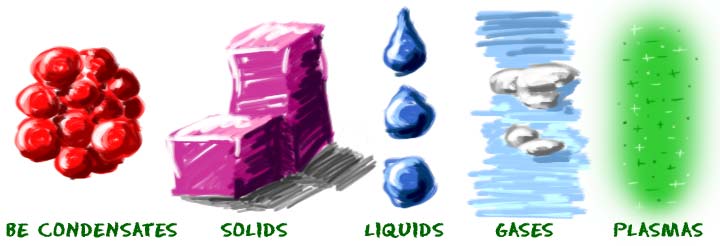
You should know about solids, liquids, gases, plasmas, and one state called the Bose-Einstein condensate (BEC). Scientists have always known about solids, liquids, and gases. Plasma was a new idea when it was identified by William Crookes in 1879. The scientists who worked with the Bose-Einstein condensate received a Nobel Prize for their work in 1995.
What makes a state of matter? It's about the physical state of the molecules and atoms. Think about solids. They are often hard and brittle. Liquids are fluidy, can move around a little, and fill up containers. Gases are always around you, but the molecules of a gas are much farther apart than the molecules in a liquid. If a gas has an odor, you’ll be able to smell it before you can see it. The BEC is all about atoms that are even closer and less energetic than atoms in a solid.
Changing States of Matter
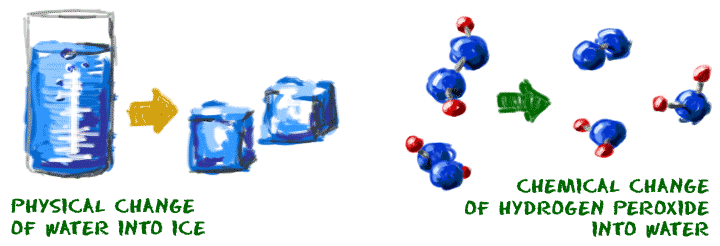
Molecules can move from one physical state to another and not change their basic structure. Oxygen (O2) as a gas has the same chemical properties as liquid oxygen. The liquid state is colder and denser, but the molecules (the basic parts) are still the same. Water (H2O) is another example. A water molecule is made up of two hydrogen (H) atoms and one oxygen (O) atom. It has the same molecular structure whether it is a gas,liquid, or solid. Although its physical state may change, its chemical state remains the same.
So you're asking, "What is a chemical change?" Let's start with a glass of pure water. If the formula of water were to change, that would be a chemical change. If you could add a second oxygen atom to a water molecule, you would have hydrogen peroxide (H2O2). The molecules would not be water anymore. The reality of creating hydrogen peroxide is more difficult.
Chemical changes occur when the bonds between atoms in a molecule are created or destroyed. Changes in the physical state are related to changes in the environment such as temperature, pressure, and other physical forces. Generally, the basic chemical structure does not change when there is a physical change. Of course, in extreme environments such as the Sun, no molecule is safe from destruction.








0 comments:
Post a Comment