Neutron Madness
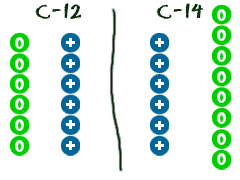 We have already learned that ions areatoms that are either missing or have extra electrons. Let's say an atom is missing a neutron or has an extraneutron. That type of atom is called anisotope. An atom is still the same element if it is missing an electron. The same goes for isotopes. They are still the same element. They are just a little different from every other atom of the same element.
We have already learned that ions areatoms that are either missing or have extra electrons. Let's say an atom is missing a neutron or has an extraneutron. That type of atom is called anisotope. An atom is still the same element if it is missing an electron. The same goes for isotopes. They are still the same element. They are just a little different from every other atom of the same element. For example, there are a lot of carbon (C) atoms in the Universe. The normal ones are carbon-12. Those atoms have 6 neutrons. There are a few straggler atoms that don't have 6. Those odd ones may have 7 or even 8 neutrons. As you learn more about chemistry, you will probably hear about carbon-14. Carbon-14 actually has 8 neutrons (2 extra). C-14 is considered an isotope of the element carbon.
Messing with the Mass
If you have looked at a periodic table, you may have noticed that theatomic mass of an element is rarely an even number. That happens because of the isotopes. If you are an atom with an extra electron, it's no big deal. Electrons don't have much of a mass when compared to a neutron or proton. Atomic masses are calculated by figuring out the amounts of each type of atom and isotope there are in the Universe. For carbon, there are a lot of C-12, a couple of C-13, and a few C-14 atoms. When you average out all of the masses, you get a number that is a little bit higher than 12 (the weight of a C-12 atom). Theaverage atomic mass for the element is actually 12.011. Since you never really know which carbon atom you are using in calculations, you should use the average mass of an atom.
Atomic masses are calculated by figuring out the amounts of each type of atom and isotope there are in the Universe. For carbon, there are a lot of C-12, a couple of C-13, and a few C-14 atoms. When you average out all of the masses, you get a number that is a little bit higher than 12 (the weight of a C-12 atom). Theaverage atomic mass for the element is actually 12.011. Since you never really know which carbon atom you are using in calculations, you should use the average mass of an atom. Bromine (Br), at atomic number 35, has a greater variety of isotopes. The atomic mass of bromine (Br) is 79.90. There are two main isotopes at 79 and 81, which average out to the 79.90amu value. The 79 has 44 neutrons and the 81 has 46 neutrons. While it won't change the average atomic mass, scientists have made bromine isotopes with masses from 68 to 97. It's all about the number of neutrons. As you move to higher atomic numbers in the periodic table, you will probably find even more isotopes for each element.








0 comments:
Post a Comment